SARS-CoV-2 IgM / IgG Rapid Test Kits are available to any licensed healthcare practitioner in the United States for diagnostic use.
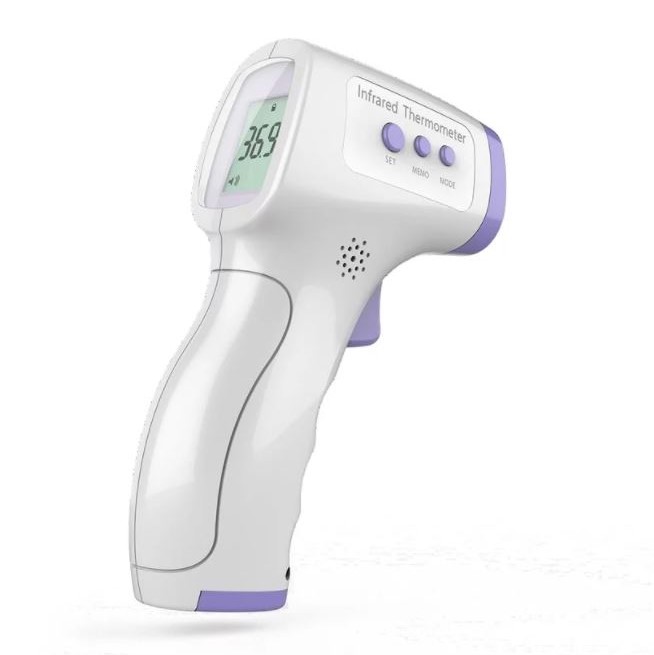
Thermometers
- Infrared Forehead Thermometer
- Measurement Mode:
Forehead mode
- Measurement Distance: 0-30mm
- Measurement Range: 89.6F ~ 107.96F
- Voltage Source: 2 x 1.5v; Battery size AAA
- Memory: Previous temperature record
- Display Screen: Backlit large screen
- Automatic Power Off 30 sec
- Dimension: 150mm* 95mm*45mm
- Weight: 146.8g
- 1 Piece
Qualification & Standard
- FDA
- CE

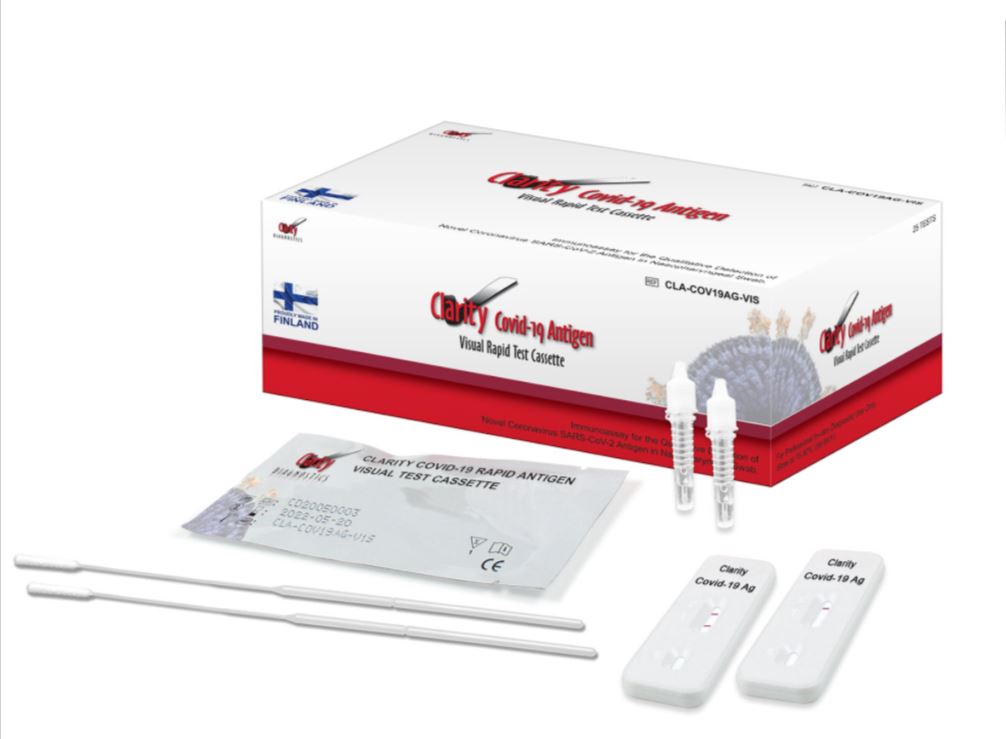
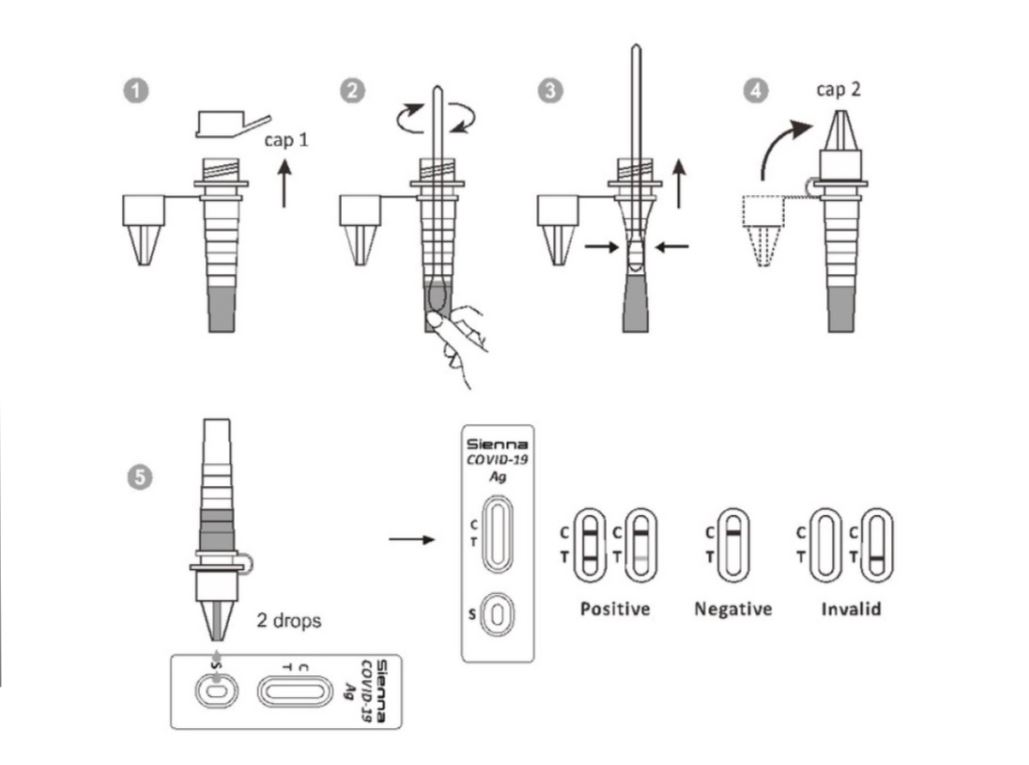
Clarity (Sienna) COVID-19 Antigen Rapid Test
The Clarity (aka Sienna) COVID-19 Antigen test can be used to test directly collected naso-pharyngeal swab specimens.
The Clarity COVID-19 Antigen test should be ordered for the detection of COVID-19 antigen in individuals who are suspected of COVID-19 by their healthcare provider and who are within six days of symptom onset.
The Clarity COVID-19 Antigen test is authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity, moderate complexity, or waived tests. This test is authorized for use at the Point of Care (POC), i.e., patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Brand | Clarity SIENNA |
Quantity | 25 per box |
Type | Nasopharyngeal, Lower Nasal |
Results Time | 10 minutes |
FDA EUA | Yes |
Sensitivity | 93.80% |
Specificity | 99.20 |
Results | Fast and Easy – Positive results as fast as 5 minutes |
Storage and Use | Test Naso-Pharyngeal Swab immediately, or store at room temperature and test within 1 hour. Naso-Pharyngeal Swab stored in a dry tube at 2-8c can be tested within 24 hours. |
Weight / Dimensions | 2lbs / 9” x 9” x 6” |
INDICAID COVID-19 Rapid Antigen Test
The INDICAIDTM COVID-19 Rapid Antigen Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first five (5) days of symptom onset. Anterior nasal swab specimens may be collected by a healthcare provider (HCP) or self-collected (by individuals 18 years of age or older, under the supervision of an HCP). Testing is limited to laboratories certified under the Clinical Laboratory Improvement.
The INDICAIDTM COVID-19 Rapid Antigen Test is intended for use by trained clinical laboratory personnel and medical and healthcare personnel in Point of Care (POC) settings. The INDICAIDTM COVID-19 Rapid Antigen Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
Brand | INDICAID |
Quantity | 25 per box |
Type | Nasopharyngeal, |
Results Time | 20 minutes |
FDA EUA | Yes |
Sensitivity | 84.40% |
Specificity | 97.00% |
Results | Fast and Easy – Symptomatic and Asymptomatic Positive results as fast as 20 minutes |
Storage and Use | Test Naso-Pharyngeal Swab immediately, or store at room temperature and test within 1 hour. Naso-Pharyngeal Swab stored in a dry tube at 2-8c can be tested within 24 hours. |
Weight / Dimensions | <1lb / 9” x 6.55” x 3.25” |

ECOTEST® COVID-19 POC Rapid Antigen Test
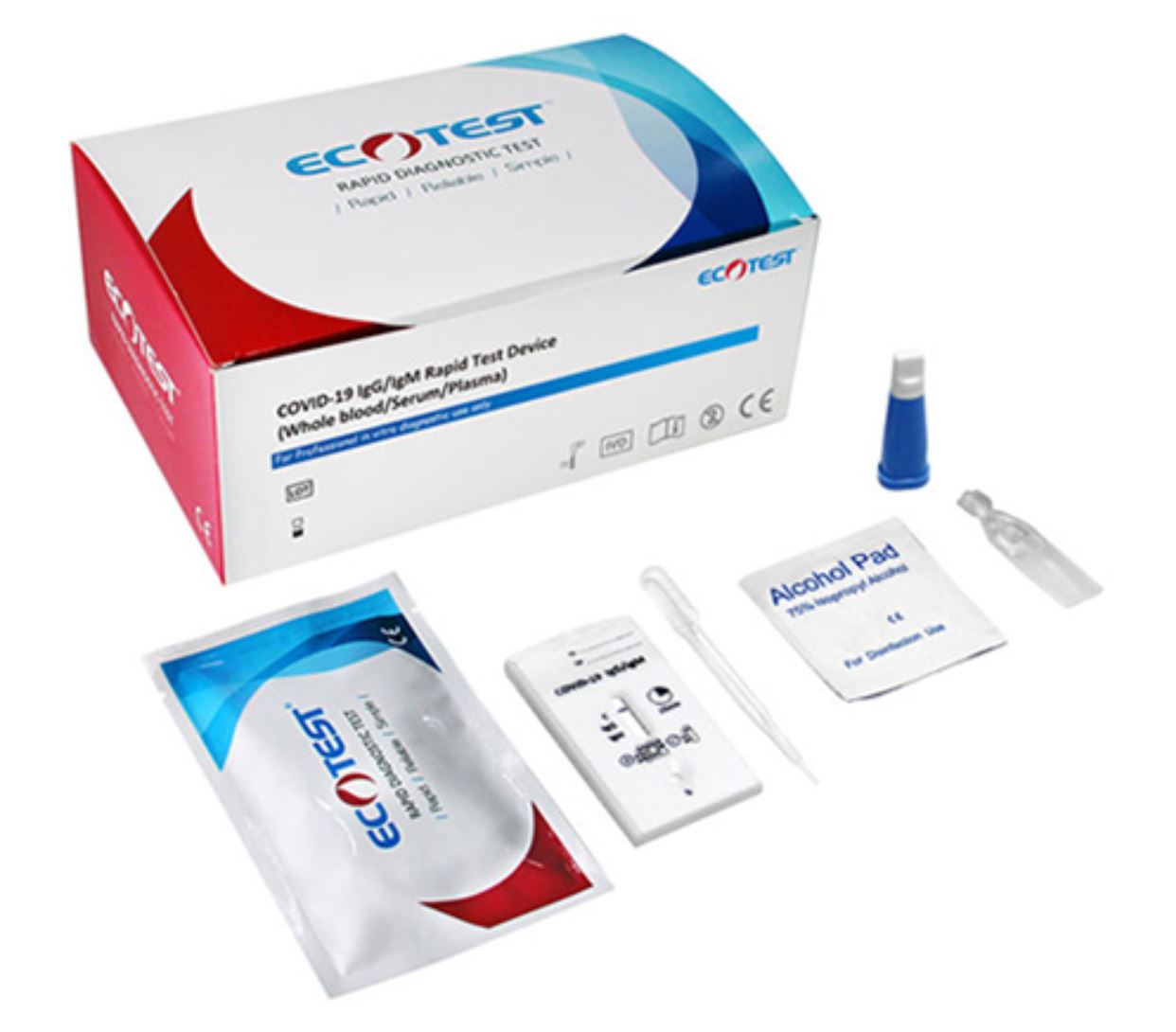
Qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood (sodium EDTA), serum, or plasma (sodium EDTA). Intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. Emergency use of this test is limited to authorized laboratories.
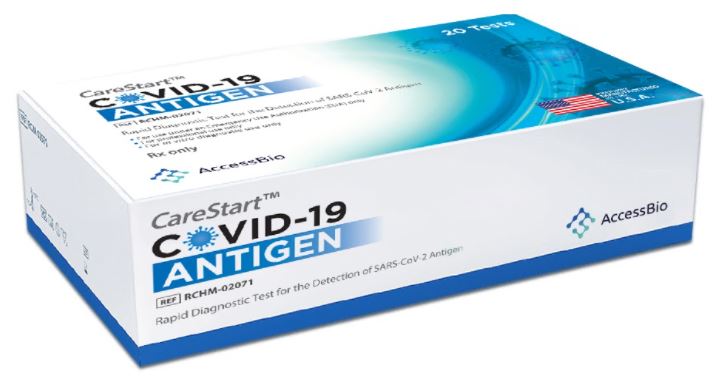
CARESTART COVID-19 POC RAPID ANTIGEN TEST
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the first five days of symptom onset or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
Carestart Antigen Test AN (20 EA/BX) The rapid antigen test is a perfect solution for testing large groups of individuals quickly, affordably, and is easy to use. Ideal for testing employees, fans, students, faculty, athletes, and more. This is an FDA EUA approved 10 Minute Point Rapid Antigen Test created for the qualitative detection of COVID-19 antigen. It is intended to aid in the rapid diagnosis of COVID-19 infections.